PeptideSynTM 多肽合成技術(shù)
Conventional solid phase peptide synthesis (SPPS) typically a long coupling time of one hour or more, extended deprotection times, multiple wash steps, and Kaiser tests to ensure complete acylation. This time-consuming long-synthesis process can be especially troublesome when synthesizing the complex peptides such as beta-amyloid. The C-terminal segment of beta-amyloid has high hydrophobicity and is subject to subsequent on-resin aggregation. The rate of amino acid acylation is heavily dependent on the properties of the coupling. For this reason, the ability to assemble quality peptides faster is highly desirable.
LifeTein's PeptideSynTM technology is a very practical platform for the synthesis of peptides for research use and pharmaceutical purposes. PeptideSynTM technology has been used for in-house testing of multiple selected biologically active peptides. These have covered a broad range including long and short peptides and peptides containing D-amino acids or pseudoproline dipeptides. The processes have used various coupling reagents such as HBTU, HATU, HCTU, ByBroP, BOP, PyBOP, and TBTU. PeptideSynTM technology allows us the flexibility to choose affordable, highly efficient coupling reagents for fast SPPS.
One study that used PeptideSynTM technology found that the synthesis of the human beta-amyloid (1-42) peptide (H-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA-OH) using HCTU as an activator required a total synthesis time of only 15 hours and produced peptides of similar purity to those generated by long synthesis. Wang-ChemMatrix, HMPB-ChemMatrix, and Wang-PSLL were compared for their ability to affect the efficiency of different synthesis procedures. The C-terminal pentapeptide was incorporated by stepwise synthesis. The coupling yields for the peptide segment were 90 to 95%. After treatment with hydrogen fluoride and purification, synthetic human beta-amyloid (1-42) peptide was obtained in an overall yield of 75-88%.
Using appropriate coupling and deprotectant reagents linked to the correct resin, LifeTein's PeptideSynTM technology provides our customers with reduced total peptide synthesis time and higher quality.
Incomplete deprotection steps and amino acid-coupling reactions can cause serious problems in the long term. Longer reaction times and increased reagent strength can solve the problem of sluggish deprotection. However, in some extreme cases, these methods are not sufficient and the protecting group cannot be removed efficiently. The side chains of some sequences render them prone to undergo self-association by hydrogen bonding. This leads to aggregation and to the formation of beta-sheet-like secondary structures that can halt peptide synthesis.
LifeTein's optimized protocol and PeptideSyn technology provide a method for circumventing the problem of difficult sequences. This technology can change peptide structure by protecting some of peptide amide bonds, giving rise to peptides containing tertiary amides at periodic intervals. This leads to better preservation of the peptide chain and to more efficient deprotection and coupling reactions. Using the Fmoc/tBu approach on our proprietary resin, the routine synthesis process of difficult sequences is improved.
Case Study: A client requested a very hydrophobic 68-amino-acid peptide (85% purity) with FITC modification at the N terminus. The peptide was successfully synthesized in 4 weeks.
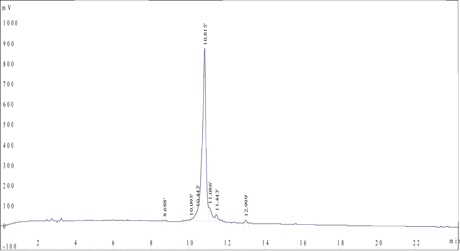
HPLC Results
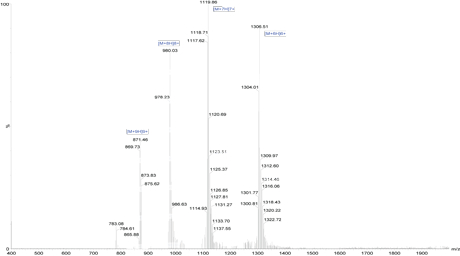
Mass Spectrometry Results
|



